A Strong Business Case for Aesthetic Practices - Sofwave
Date
Written by
Rana Bassal
Revised by
Michael H. Gold, MD
For plastic surgeons, dermatologists, and medical spas, the decision to invest in an energy-based medical aesthetic device is fundamentally driven by a platform's ability to maximize profitability, minimize risk, and capitalize on evolving consumer demand. Sofwave Medical Ltd.'s Synchronous Ultrasound Parallel Beam (SUPERB™) technology presents a superior business offering by excelling across operational, financial, and clinical metrics, distinguishing itself decisively from competing modalities.
Financial Stability and Operational Efficiency
A medical aesthetics device’s business case begins with financial viability and seamless integration into a busy clinic environment. Sofwave’s model is built for reliable scaling and high throughput:
Robust Corporate and Margin Health: Sofwave achieved a significant milestone in Q2 2025 by reporting its first-ever net income of $1.5 million on $21 million in revenue. The gross margin reported was exceptionally high at 75.5%. The company actively maintains minimum advertised pricing protection to ensure the treatment is viewed as premium, allowing providers to maintain high gross margins on the procedure, making it the "treatment of choice" among multiple options.
High Recurring Revenue: The business model relies on the sale of pulses, which constituted 40% of the company's total revenue in Q2 2025, and recurring revenue grew 53% year-over-year. This demonstrates that patients are consistently returning for treatments, guaranteeing a sustained income stream for the purchasing clinic.
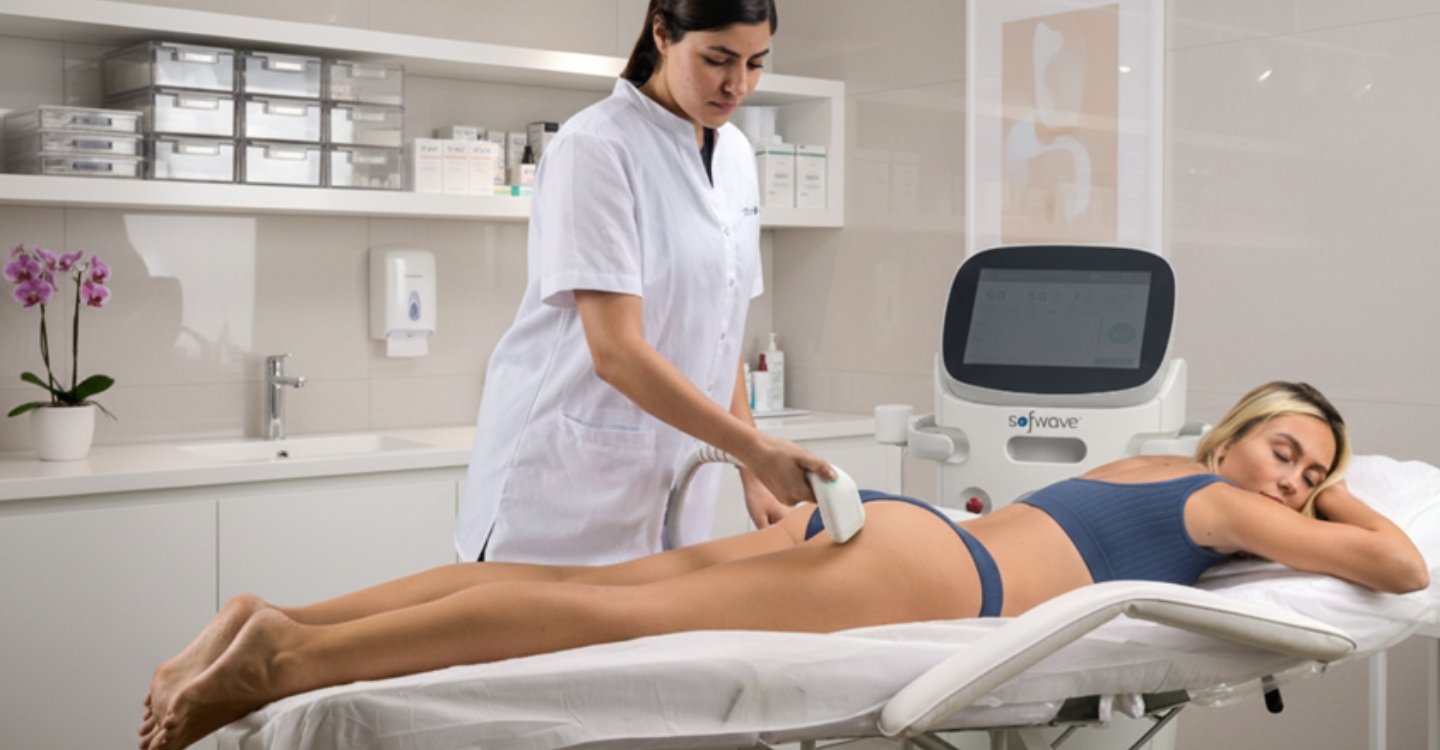
Rapid Throughput and Minimal Downtime: Time efficiency is critical for maximizing a practice’s earning potential. A full-face Sofwave treatment takes about 45 minutes. Since the procedure involves minimal downtime, patients can immediately return to their normal daily activities. This speed is "perfect for busy schedules" and significantly boosts clinic throughput.
As Dr. Paul Jarrod Frank, MD, confirms, "You can return to your normal daily activities immediately after the procedure". This speed is "perfect for busy schedules" and significantly boosts clinic throughput.
Ease of Delegation and Use: The device is described as "exceptionally easy to use and quick to learn
Reliability: The SUPERB™ handpiece utilizes a proprietary solid-state energizer module with no moving parts or optics, contributing to its high durability and reliability.
Clinical Superiority and Risk Mitigation
Sofwave provides a competitive advantage by offering broad clinical efficacy across multiple indications, paired with a high safety profile that mitigates liability risks associated with competing technologies.
Sofwave's Unique Safety and Efficacy Profile (Bullet Points)
Precision and Depth: SUPERB™ technology delivers non-focused ultrasound beams, precisely targeting and heating the mid-dermal layer at 1.5 mm to stimulate neocollagenesis and neoelastogenesis.
Epidermal Protection: The device includes Sofcool™ Technology and real-time temperature monitoring, which effectively protects the epidermal layer from thermal damage.
Minimal Pain: The procedure is generally well-tolerated, often requiring only topical anesthesia.
Broad Skin Type Application: The non-invasive ultrasound method is safe for all skin types and tones (including Fitzpatrick types II–IV tested in clinical studies) as it is not affected by melanin, avoiding the risks of hyperpigmentation common with other heat/light-based treatments on darker skin.
Versatile FDA Clearances: Sofwave is FDA-cleared for multiple uses, including lifting the eyebrow, neck, and submental tissue, improving facial lines and wrinkles, improving skin laxity on the upper arms, short-term improvement in cellulite, and treatment of acne scars.
Mitigating Risks Associated with Competitors
The safety of Sofwave directly contrasts with the risks and limitations of common aesthetic devices, offering clinics a clear liability advantage:
RF Microneedling (RFMN): RFMN involves the use of fine needles and heat delivery beneath the skin. The FDA has issued safety warnings regarding RF microneedling devices due to reports of serious adverse events, including burns, scarring, fat loss, disfigurement, and nerve damage. Practices using RFMN are explicitly advised to review marketing materials and update consent forms to disclose these risks. Sofwave’s entirely non-invasive ultrasound delivery bypasses these risks entirely, as reflected by the reported absence of adverse events in clinical trials.
Fractional Ablative CO2 Lasers (FACL): FACL, while effective, is associated with significant downtime. FACL procedures require extensive pain management, with a consensus among experts favoring topical anesthetic creams, nerve blocks, and oral analgesics. Furthermore, FACL is primarily ideal for patients with fair skin (Fitzpatrick I–II), while Sofwave is safe and effective across a broader range of skin types, including skin of color.
Capturing High-Growth Aesthetic Market Segments
Sofwave is strategically aligned with the key market trends driving consumer demand in 2025, ensuring high patient conversion rates for B2B partners:
The GLP-1 Phenomenon: The rise of weight loss medications (GLP-1s) is creating a new class of aesthetic patients, with 63% of those seeking aesthetic services being first-time patients. Their primary concern is skin laxity, loose skin, and sagging—a condition often termed "Ozempic face". Sofwave provides the ideal non-surgical lifting solution to address this specific, high-growth demographic.
The Shift to Natural Rejuvenation: The industry is moving away from "overfilled" aesthetics. Dermal filler use decreased by 31% in 2024. In contrast, the market segment demanding skin lifting grew by 39% year-over-year. Sofwave’s mechanism, which stimulates the body’s natural production of collagen and elastin, aligns perfectly with the demand for subtle, natural-looking results and the "Quiet Luxury Face" trend.
This trend is also fueled by concerns over long-term injectable use, as dermatological surgeon Dr. Patricia Wexler, MD, notes regarding muscles treated with Botox: "If used regularly, over a prolonged period, without interruption, eventually the muscle will atrophy from lack of use". Sofwave provides the necessary deep dermal remodeling without the risk of muscle atrophy.
Strong Brand Awareness and Demand Generation: Sofwave invests heavily in national branding to drive patient demand directly to its provider partners. The brand achieved a 34% "share of voice" across competitive keywords (like GLP-1 and skin laxity) in Q2 2025, ensuring that when consumers search for solutions, Softwave appears prominently.
| B2B Business Advantage | Sofwave’s Solution | Competitive Distinction |
|---|---|---|
| Maximize Throughput | Quick treatment time (30–45 minutes) with zero downtime. | Delegatable by staff, freeing physician time. |
| Reduce Liability & Risk | Non-invasive ultrasound with integrated cooling. | Avoids severe risks (burns, scarring, fat loss) associated with RF microneedling, which led to FDA safety warnings. |
| High ROI & Recurring Revenue | 75.5% Gross Margin; 40% revenue from pulses. | Predictable, long-term income stream post-device sale. |
| Address New Demand (GLP-1) | Provides necessary non-surgical lifting solutions. | Directly solves the primary aesthetic concern (skin laxity) for the fastest-growing segment of new patients. |
Strategies for Optimal Practice Utilization (List)
Target the New Patient Demographic: Actively market Sofwave’s lifting capabilities to patients experiencing skin laxity following rapid weight loss from GLP-1 medications.
Optimize Staff Utilization: Utilize the device's speed and delegatability to allow trained staff (e.g., nurse practitioners or physician assistants) to manage high-volume Sofwave and Pure Impact VIP™ procedures, maximizing the number of patients treated daily.
Leverage Versatility: Capitalize on the device's numerous FDA clearances, promoting it not just for face/neck lifting, but also for acne scar treatment in patients with diverse skin types, a capability proven safe and effective in clinical studies.
Emphasize Low Risk: Use Sofwave’s excellent safety profile (no downtime, non-invasive, safe for all skin tones) as a key selling point against competitors known for painful procedures (Ultherapy) or serious complications (RF microneedling).
Sofwave provides B2B partners with a highly efficient platform that aligns with the major trend toward natural rejuvenation, patient safety, and minimal downtime. Investing in Sofwave is like acquiring a versatile, high-speed, multi-tool designed not only for today's market but specifically engineered to manage the complex demands being driven by new aesthetic trends like the GLP-1 phenomenon.
References
- Scott Hollenbeck, MD, 2024 Plastic Surgery Statistics Report, 2024 https://www.plasticsurgery.org/documents/news/statistics/2024/plastic-surgery-statistics-report-2024.pdf
- Ruthie Amir, MD, A novel approach to treating fine lines and wrinkles of the face using Synchronous Ultrasound Parallel Beam Technology SUPERB™, 2020 https://api.sofwave.com/app/uploads/2024/12/PB00030-4_White-PaperFINAL.pdf
- Earnings call transcript: Sofwave Medical Q2 2025 shows strong revenue growth, 2025 https://www.investing.com/news/transcripts/earnings-call-transcript-sofwave-medical-q2-2025-shows-strong-revenue-growth-93CH-4170665
- Ariel B. Brown, Courtney H. Rawitscher, and Craig F. Teller, Neocollagenesis to Reduce Skin Laxity: A Review of the Mechanisms and Efficacies of Modern Devices, 2025 https://ascpd.org.au/wp-content/uploads/ascpd-Cosmeceuticals-for-Hyperpigmentation-10.11-Neocollagenesis-to-Reduce-Skin-Laxity.pdf
- 4 Effective Non-Surgical Neck Tightening Procedures To Try in 2025, 2025 https://springstderm.com/4-effective-non-surgical-neck-tightening-procedures-to-try-in-2025/
- Hallie Gould, 13 Botox Alternatives for Smoother Skin and Similar Anti-Aging Results, 2025 https://www.byrdie.com/botox-alternatives
- Michael H Gold, Julie Biron, Efficacy and safety of high-intensity, high-frequency, non-focused ultrasound parallel beams for facial skin laxity, 2023 https://pubmed.ncbi.nlm.nih.gov/38031530/
- Kanjana Boonchoo, Wichai Hongcharu, The Efficacy and Safety of a Single Treatment of High-Intensity, High-Frequency, Non-Focused Ultrasound Parallel Beams for Facial Acne Scars in Asian Patients: A Preliminary Study, 2024 https://api.sofwave.com/app/uploads/2025/04/Dr-Wichai-Article.pdf
- Marisa Martino, Sofwave Therapy Reviews: Real Reviews from Real People, 2022 https://skinneymedspa.com/sofwave-therapy-reviews/
- Amanda Montell, 9 Long-Term Effects of Botox You Should Know, 2024 https://www.byrdie.com/long-term-effects-of-botox